Whether you’re processing medication claims, working in pharmacy billing, or managing healthcare inventory, you’ve likely crossed paths with something called an NDC code. Short for National Drug Code, this is more than just a string of numbers on a label—it’s a federally regulated identifier that plays a vital role in drug identification, billing, and compliance in the U.S. healthcare system.
If you’ve ever had a claim rejected due to a wrong NDC, you already know how costly a small error can be. But managing NDC codes properly is about more than reimbursement—it’s about accuracy, safety, and compliance.
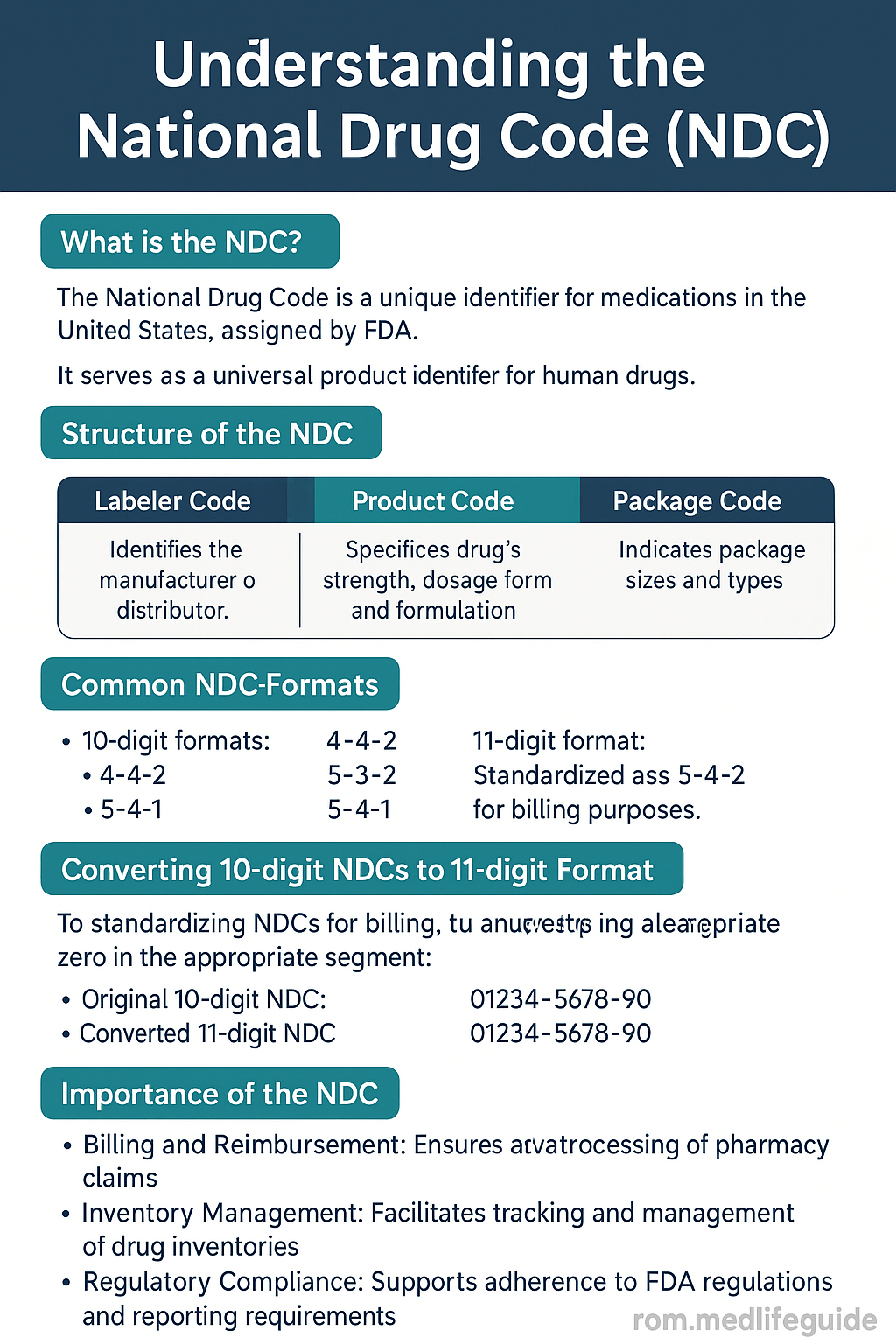
Breaking Down the NDC Code Structure
Every FDA-approved medication is assigned a unique NDC number, which is either 10 or 11 digits long depending on the format. This number is split into three segments:
- Labeler Code – Identifies the company that manufactures or distributes the drug.
- Product Code – Specifies the drug’s strength, dosage form, and formulation.
- Package Code – Describes package type and quantity.
For example, an NDC code might appear as 12345-6789-01:
12345is the labeler6789identifies the product01denotes the package size
- In CMS1500 form, the NDC code is available in the shaded portion of the line item field 24A-24G as shown in below image,
- In the UB04 form, the NDC code is available in field 43 as shown in the below image,
- As you can see in both images, when an NDC code is billed on a claim form, it should have a format that includes the NDC Qualifier, NDC code, NDC unit of measure & number of NDC units.
1) NDC Qualifier: “N4” Qualifier needs to bill with the NDC code.
2) NDC Code: Uniques 11 digits number assigned to each drug code.
3) NDC unit of Measure: There are 4 units to measure drugs,
- UN (Unit) – Powder-filled vials for injection (needs to be reconstituted), pellet, kit, patch, tablet, and device.
- ML (Millilitre) – Liquid, solution, or suspension.
- GR (Gram) – Ointments, creams, inhalers, or bulk powder in a jar.
- F2 (International Unit) – Products described as IU/vial, or micrograms.
4) NDC Units: These define the quantity of the drugs.
How to Verify an NDC Code (Without Guessing)
Verification is key, especially for high-cost or specialty medications. These are a few of the most trusted NDC code lookup resources:
- FDA’s NDC Directory – Free and official
- DailyMed by NIH – Includes drug label data and usage information
- Drugs.com – Easy-to-use interface for quick lookups
- Commercial databases – Like First Databank or Medi-Span, for enterprise-level verification and automation
Human insight opportunity: If you have experience comparing free vs. paid tools, you could offer a unique angle or share pros/cons based on real usage.
Best Practices for Managing NDC Codes in Your System
NDC code management can become chaotic without a strategy. Here’s how to stay on track:
- Standardize formats: Many systems require 11-digit NDCs. Convert 10-digit versions accordingly using a leading-zero format.
- Automate updates: Use software that syncs with FDA or commercial databases to prevent outdated entries.
- Train your team: Mistakes often stem from human error. Staff education pays off.
- Log changes and version history: Keep a record of updates to avoid audit headaches later.
Top Software Tools for Managing NDC Codes
Whether you’re in a small practice or a large hospital, the right software can make a major difference. Here are some of the best-reviewed tools for NDC code tracking and billing:
- eClinicalWorks – Good for small to mid-sized practices
- NextGen Healthcare – Integrates NDCs directly into billing workflows
- Redox – Ideal for EHR integrations
- First Databank (FDB) – Enterprise-grade solution with drug intelligence
- McKesson – Pharmacy-focused with excellent support
When evaluating tools, look for automated formatting, alerts for discontinued codes, and user access controls to prevent unauthorized edits.
How to Handle NDC Code Changes
Drug manufacturers sometimes update packaging, discontinue products, or reassign NDCs. Keeping up with those changes is crucial.
Tips for Staying Current:
- Subscribe to FDA’s NDC updates
- Set quarterly reviews in your system
- Partner with software vendors that automate NDC refreshes
- Flag high-volume or high-risk drugs for priority review
Common Mistakes (And How to Avoid Them)
Even experienced teams make errors with NDC codes. Here are some of the most frequent ones:
- Using an NDC for the wrong package size
- Not converting 10-digit codes to HIPAA-compliant 11-digit format
- Over-relying on barcodes instead of validated NDC entries
- Keeping inactive or obsolete codes in your system
Pro tip: Build a checklist or internal audit process to catch errors before claims go out.
Alternatives to NDC Codes: Are There Other Systems?
While the NDC is king in U.S. healthcare, globally there are a few alternatives:
- GTIN (Global Trade Item Numbers) used in supply chain logistics
- ATC (Anatomical Therapeutic Chemical) codes in Europe for clinical categorization
- UPC codes used more commonly in retail than medical settings
However, for billing, tracking, and regulatory use in the U.S., NDC remains the gold standard.
58 Modifier in Medical Billing
Modifier 58 is a critical billing tool used to indicate a staged…
Subscriber, Dependent, and Spouse in Health Insurance
In medical billing and insurance, the terms subscriber, dependent, and spouse have…
What is Coinsurance in Health Insurance?
Coinsurance is the percentage of a medical bill you pay after meeting…
What are deductibles in health insurance?
Introduction: A deductible is the amount of money a patient has to…
What is Copay (PR-3) in Health Insurance?
A copay is a fixed amount that a patient is required to…
Authorization In Medical Billing And Its Types
Authorization is key in healthcare. It helps patients get the care they…